Abstract
Introduction The Human T-cell leukemia virus type 1 (HTLV-1) is an endemic virus present in distinct areas of the world such as Japan, the Caribbean basin, and Latin America. This virus is associated with adult T-cell leukemia-lymphoma (ATL), a hematological disease with dismal outcomes. To date, there is not established standard treatment for ATL, and it is often unstructured in the real-world setting. Moreover, few attempts have been conducted to describe the clinical outcomes across countries. Therefore, we aimed to compare the clinical features, treatment approaches, and outcomes between worldwide endemic regions.
Methods We designed a retrospective cohort study of patients from Latin America, the Caribbean, Japan, and the United States (USA) managed at 18 academic centers from 1980 to 2015. Patients with a pathology confirmation of T-cell lymphoma and a positive serum test for HTLV-1 were included in the study. Patient data was manually abstracted from medical records in a standardized form. All individuals from the Caribbean were treated at USA centers. Overall survival (OS) was defined as the time from diagnosis to death from any cause, while progression-free survival (PFS) was defined as the elapsed time from diagnosis to relapse or disease progression or death from any cause. The lowest median follow-up (reverse Kaplan-Meier method) from these regions was used to censored and compare survival outcomes. The Kaplan-Meier and the Log-rank test were employed to estimate and compare survival probabilities between the regions. The Cox regression was used to model the effect of the regions on OS. Missing values were assigned a missing category in the models. We present our results with adjusted Hazard ratios (HRs) and 95% confidence intervals (CIs).
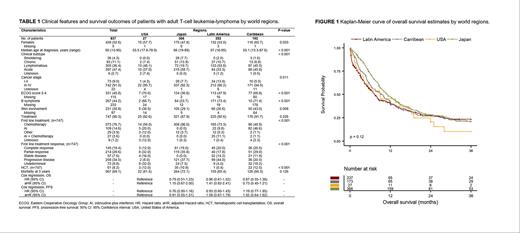
Results A total of 837 patients were identified, 366 (44%) from Japan, 252 (30%) from Latin America, 192 (23%) from the Caribbean, and 27 (3%) from the United States (USA). Table 1 shows the demographic, clinical, and treatment features by world regions. Advanced cancer stage (III-IV) at diagnosis was common among all regions (86-95%). However, acute ATL was the most common presentation in Japan (59%), while lymphomatous ATL was more frequent in Latin America (53%), the Caribbean (45%), and the USA (48%; p<0.001). Japanese patients presented with a higher median age (66 vs. 53-57 years, p<0.001) and lower frequency of B symptoms at diagnosis (24% vs. 67-73%, p<0.001). Caribbean and USA patients had poorer performance status (69% and 70%, respectively; p<0.001) and higher skin involvement (43% and 39%, respectively) compared to Japanese (ECOG: 37%, skin: 29%) and Latin American (ECOG: 48%, skin: 27%; p=0.008) patients. Frontline treatment was administered to 90% (n=747) of all individuals (range 88-93% across regions). However, treatment patterns varied by world region. Chemotherapy was the most common approach in Japan (96%), Latin America (73%), the USA (56%), and the Caribbean (49%). Zidovudine plus interferon was not used in Japan but this regimen or combined with chemotherapy was frequent in Latin America (21%), the Caribbean (48%), and the USA (20%). Overall response rates (complete and partial response) ranged from 38% in Latin America to 55% in Japan. Hematopoietic cell transplantation was used in 11-13% of Japanese, Caribbean, USA patients; only one Latin American patient received a transplant. The 3-year OS and PFS rates were 21% and 8% for all patients. OS and median OS at 3 years was dismal and similar between regions (10% - 9 months in the USA, 21% - 10 months in the Caribbean, 21% -11 months in Japan, and 24% - 7 months in Latin America, p=0.118) (Figure 1). Similarly, PFS and median PFS at 3 years were poor in the Caribbean (2%, 4 months), Japan (9%, 7 months), and Latin America (10%, 5 months). All USA patients experienced an event before 3 years with a 6-month median PFS. When using USA as a reference category, Japan, the Caribbean, and Latin America were not associated with worse outcomes in the multivariate Cox analyses at 3 years.
Conclusion To our knowledge, this is one of the largest cohort of patients with ATL in the real-world setting. Our findings suggest that key clinical features and frontline treatment approaches varies between world regions. However, survival outcomes were similar and dismal for all regions at 3 years, suggesting the worldwide need to develop new drugs and regimens to treat this condition in endemic regions.
Disclosures
Utsunomiya:Meiji Seika Pharma: Honoraria; JIMRO: Consultancy; Bristol-Myers: Honoraria; Otsuka Medical Devices: Consultancy. Takamatsu:Chugai Pharmaceutical: Research Funding; AstraZeneca: Honoraria; Sanofi: Honoraria; Janssen Pharmaceutical: Honoraria. Ishitsuka:Takeda: Honoraria; Astellas: Honoraria; Eisai: Honoraria; Nippon-Shinyaku: Honoraria; Meiji Seika: Consultancy, Honoraria; Otsuka: Honoraria; Pfizer: Honoraria; OQVIA: Honoraria; Otsuka: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; Yakult: Honoraria; BMS: Honoraria; Celgene: Honoraria; Kyowa Kirin: Honoraria, Research Funding; Chugai: Honoraria; AbbVie: Honoraria; CSL Behring: Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Ono: Honoraria, Research Funding. Suzumiya:Chugai-Roche: Consultancy, Honoraria, Research Funding; Dainihon-Sumitomo: Honoraria; Eisai: Honoraria, Research Funding; AstraZeneca: Honoraria; Bristol Myers Suquibb: Honoraria; Kyowa-Kirin: Consultancy, Honoraria, Research Funding; Jansen: Honoraria; Eli Lill: Honoraria; AbbVie: Honoraria; Nihonshinyaku: Honoraria; Novartis: Honoraria; Otsuka: Consultancy, Honoraria; SymBio: Honoraria; Takeda: Honoraria; Zenyaku: Consultancy. Tamura:AC Medical: Consultancy; Symbio Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Eisai Co., Ltd: Membership on an entity's Board of Directors or advisory committees, Other: DSMB; Ono Pharmaceutical Co., Ltd: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal